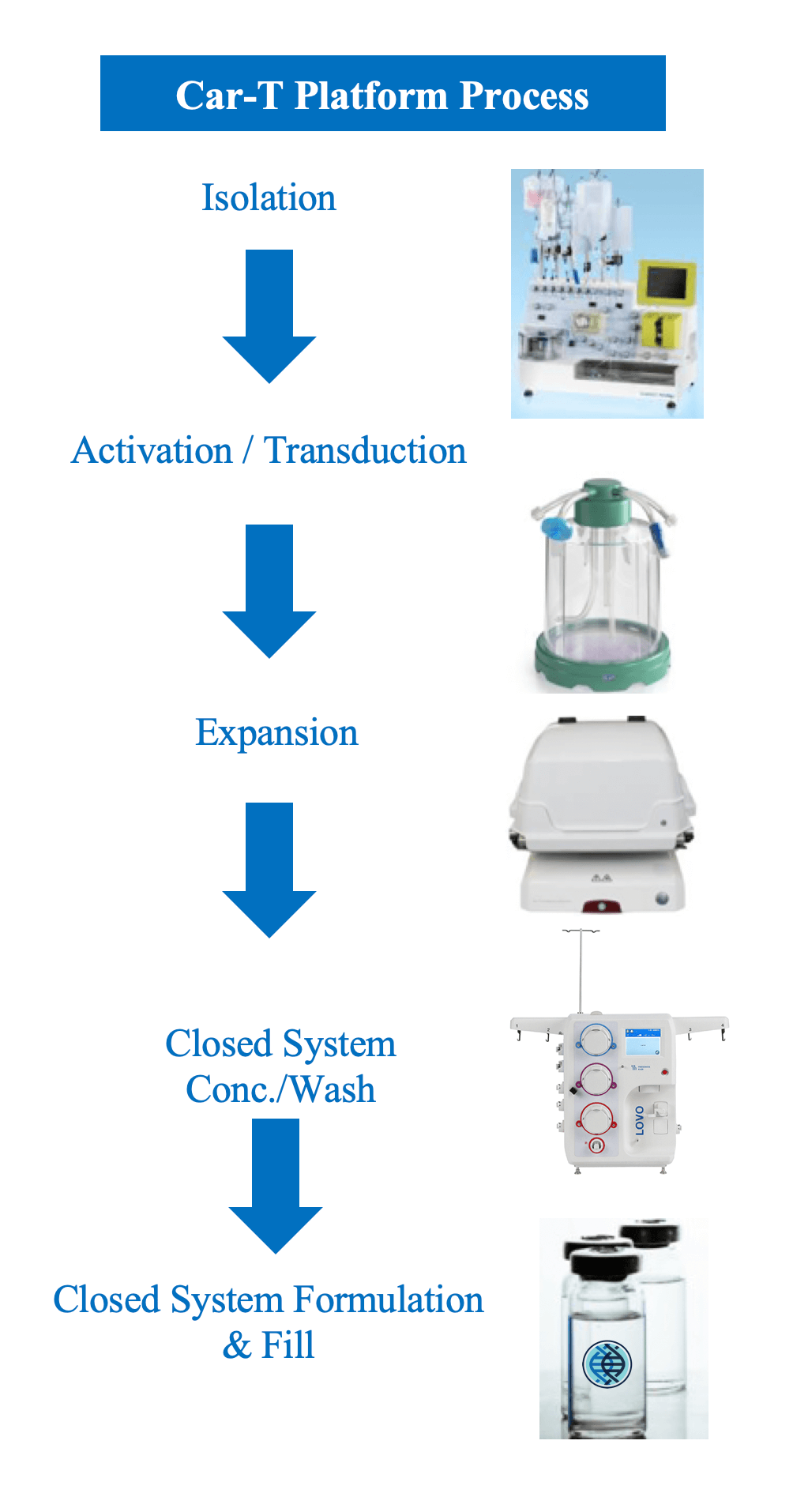
Closed-Process CAR-T
Platform
Our best-in-class service platforms enable cell and gene therapies to be developed, manufactured, and released faster and with greater predictability
We have been manufacturing CAR-T products since 2014. Our Closed Process CAR-T Cell Therapy Platform provides advanced therapy companies with fully integrated CAR-T production capabilities that support groundbreaking advances in the field of cell and gene therapies and help speed these innovative treatments to global markets – and patients – with greater predictability.
Platform Features
- A honed two-tier development paradigm (EDP/LDP) offering a closed system of end-to-end integrated solutions.
- Regulatory and technical expertise, with dedicated process and analytical development teams.
- A state-of-the-art GMP manufacturing facility that offers enhanced capacity and scheduling flexibility to meet customer timelines.
- In-stock raw materials and consumables – ready for use – with established batch records.
- Full in-process and release testing, plus complete quality control and characterization services.
- Choice of two modalities depending on dose requirements, using a list of pre-evaluated equipment, technologies and materials with developed closed processes for different unit operations.