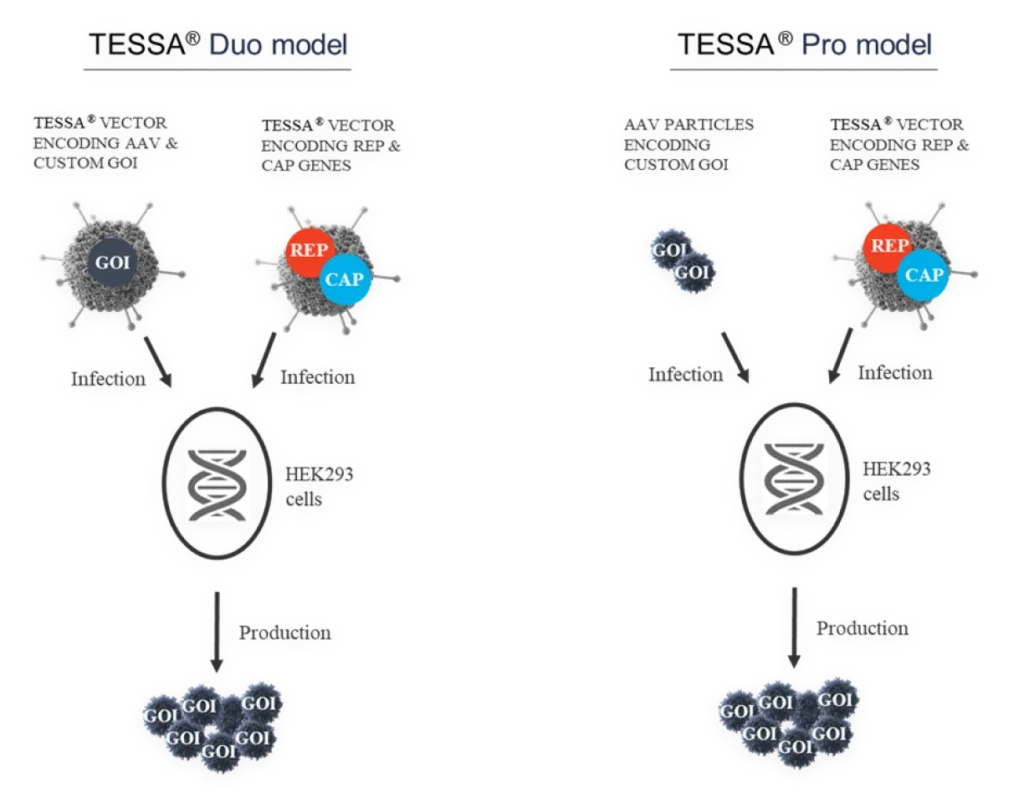
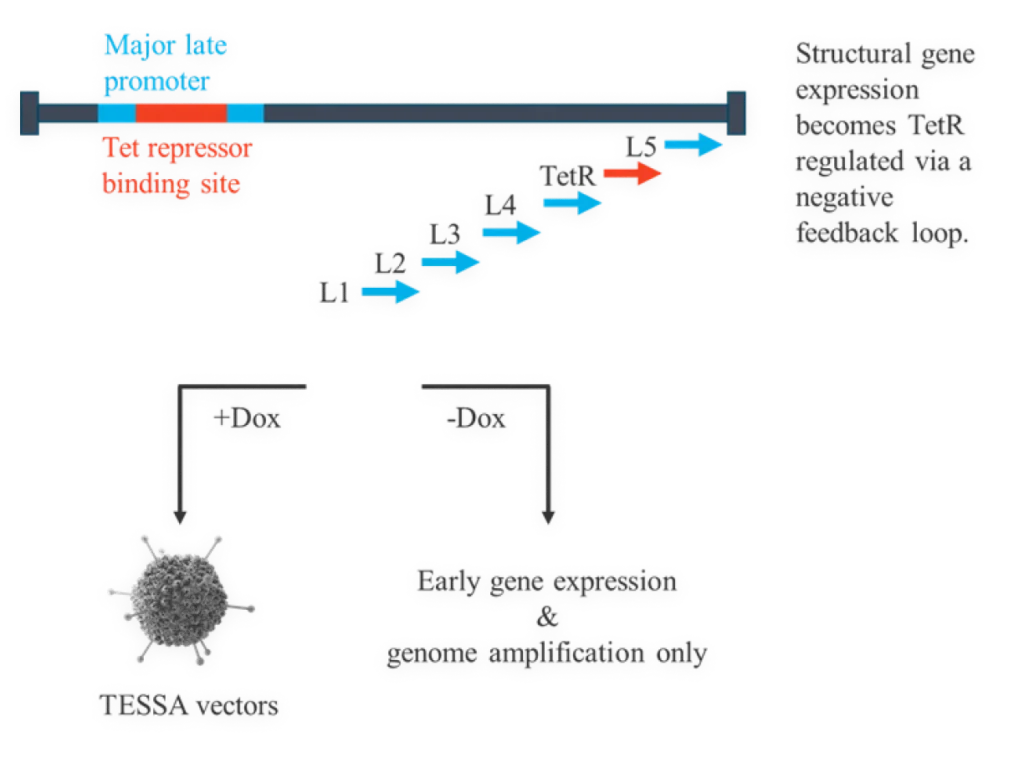
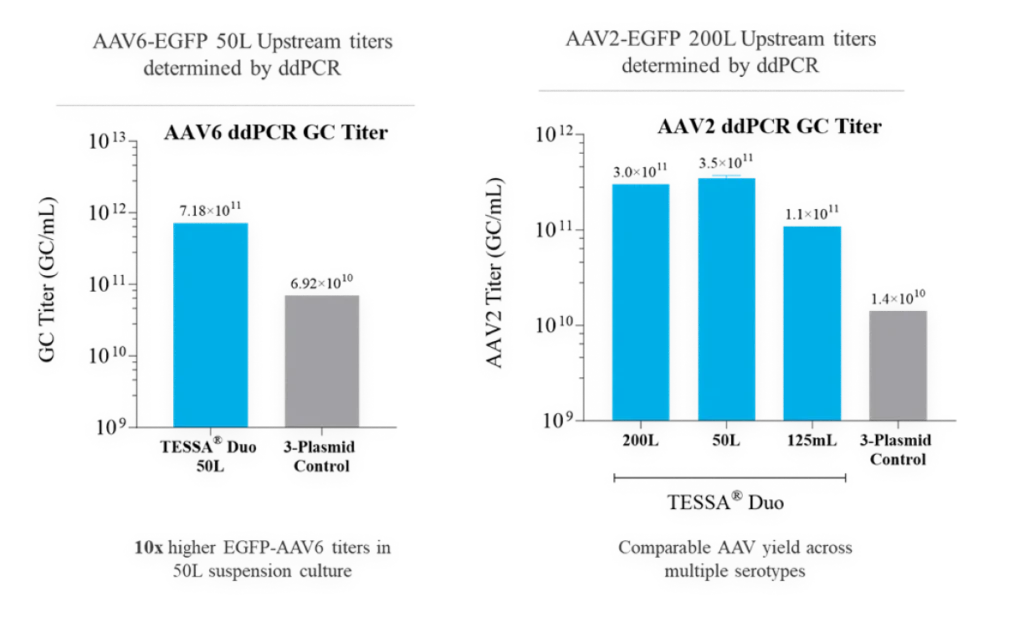
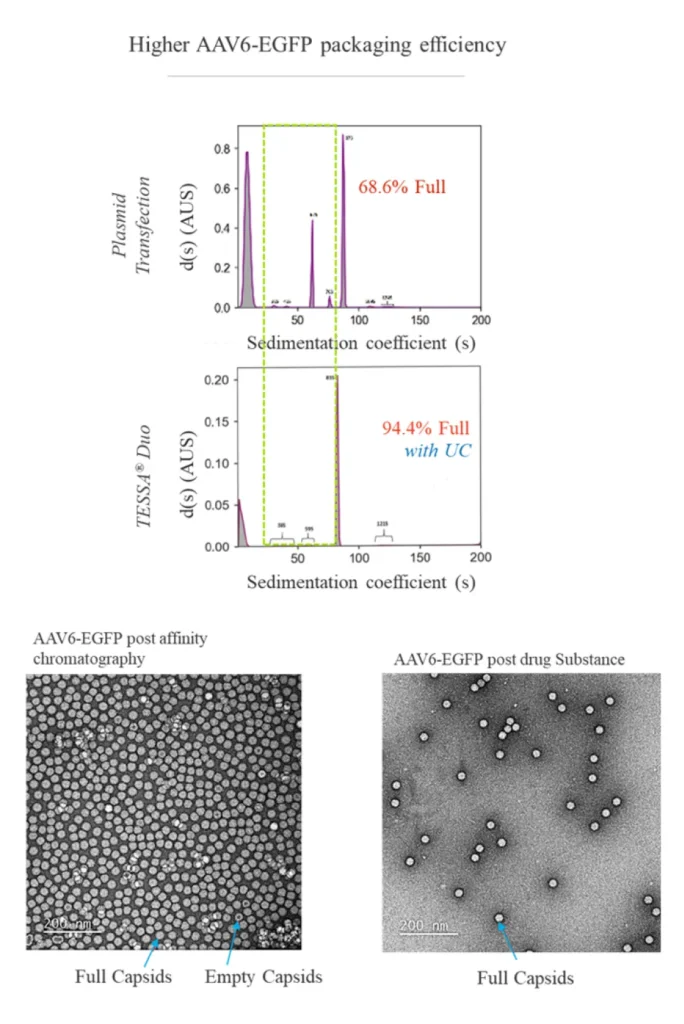
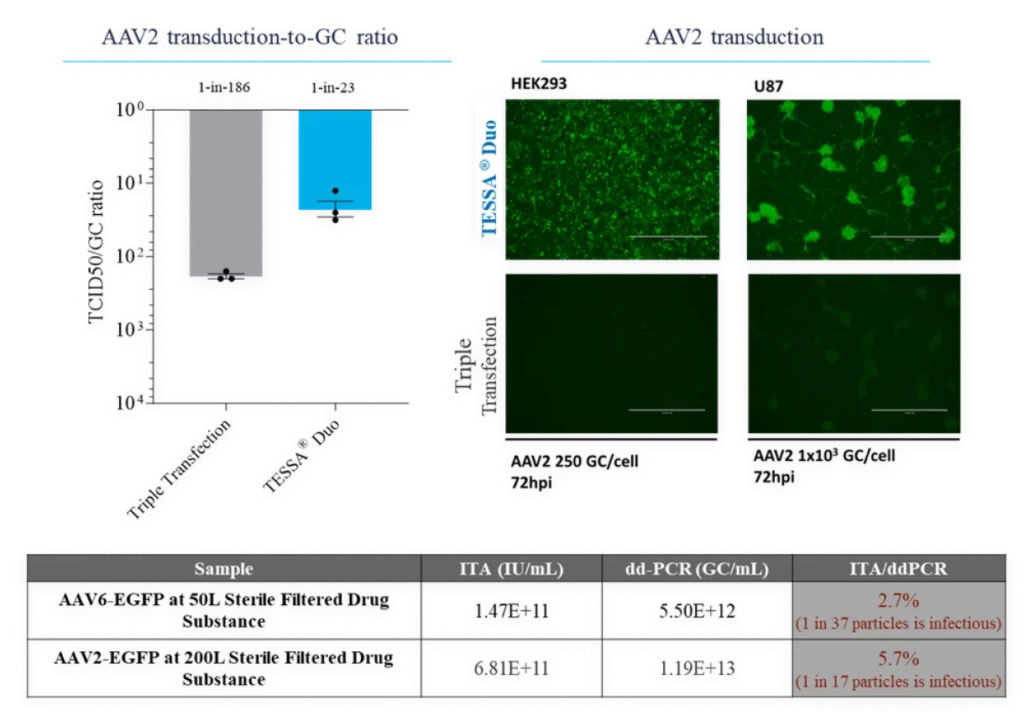
TESSA® vectors take advantage of the adenoviral lifecycle, which contains two temporal phases: early and late. The early phase provides all the adenoviral help needed for high fidelity, high titer AAV manufacture. The late phase leads to adenovirus contamination.
We’ve engineered supremely tight regulation of the adenoviral late region, which is wholly responsible for producing adenovirus structural proteins. Using TESSA® technology, full early region expression, which provides all the help needed for AAV production, is enabled. However, the late region gene expression is turned off, meaning there is no adenoviral structural protein production.
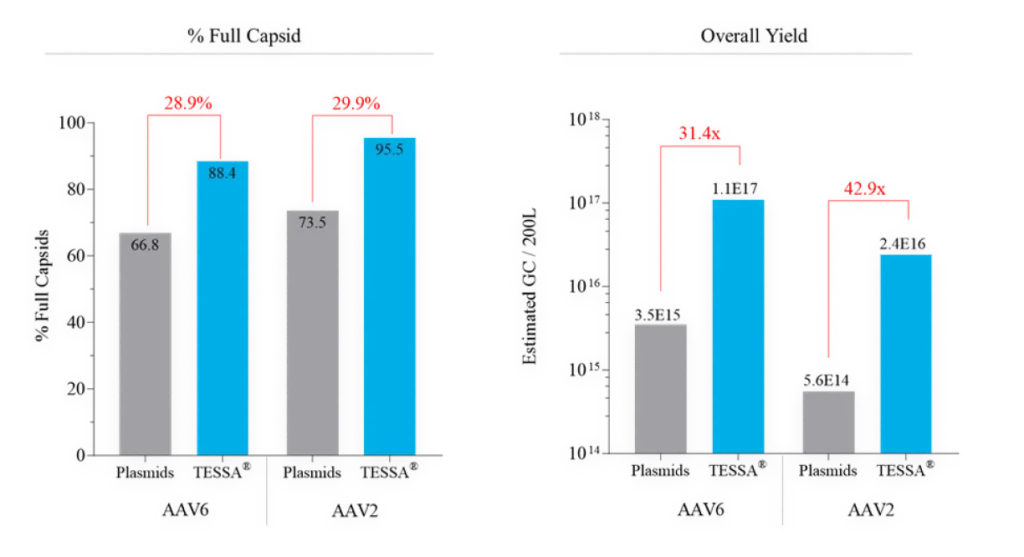
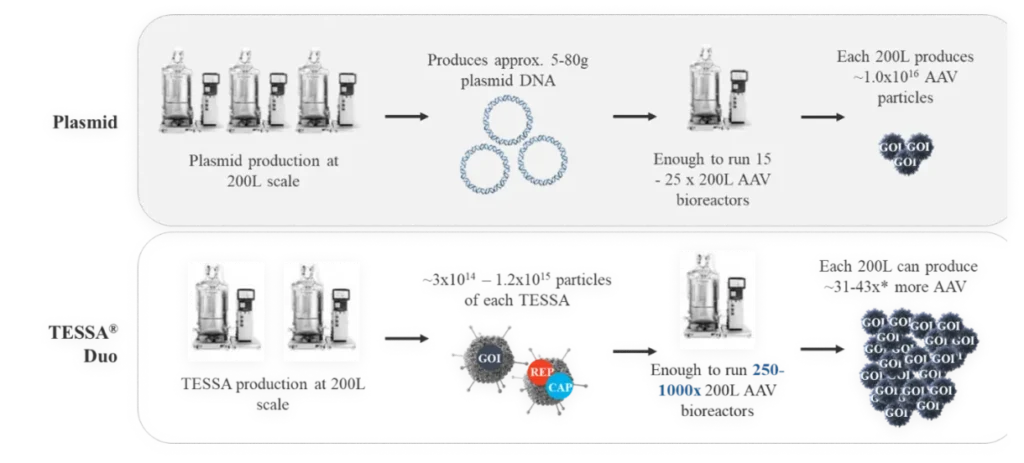
This tightly represses adenovirus production during an AAV manufacturing run, while increasing recombinant AAV (AAV) yield, packaging efficiency and infectivity compared to both Ad5 helper plasmid or wild-type adenovirus. Stable integration of AAV Rep and AAV Cap into the TESSA® vector allows efficient and simultaneous delivery of all the necessary components for AAV replication in a single agent.