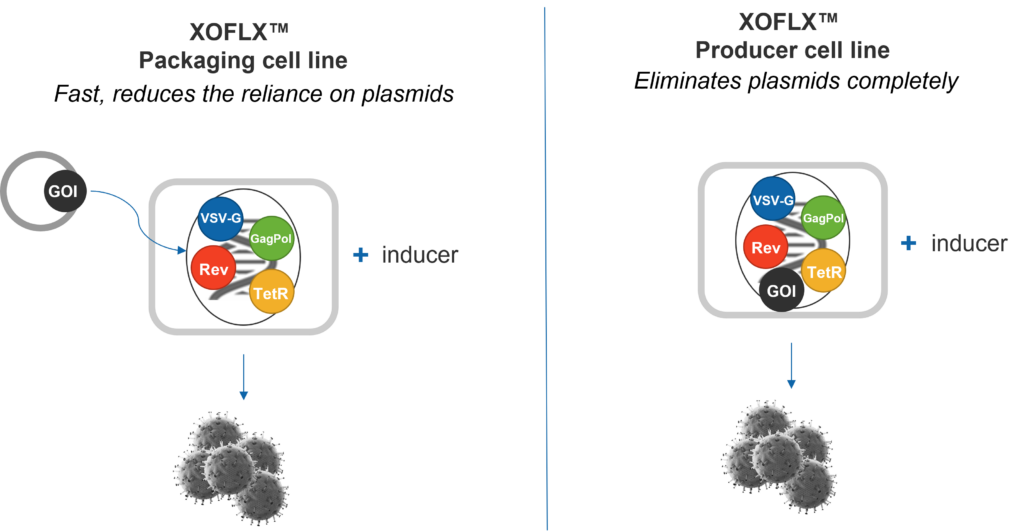
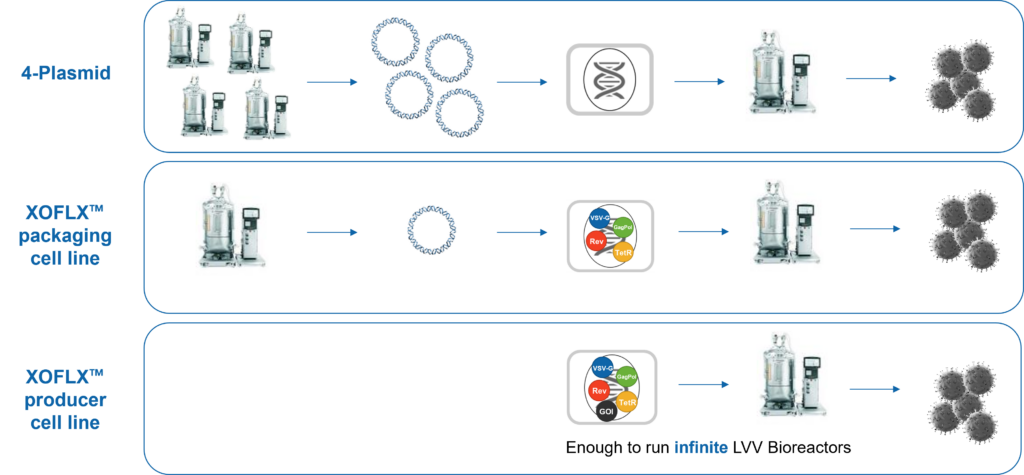
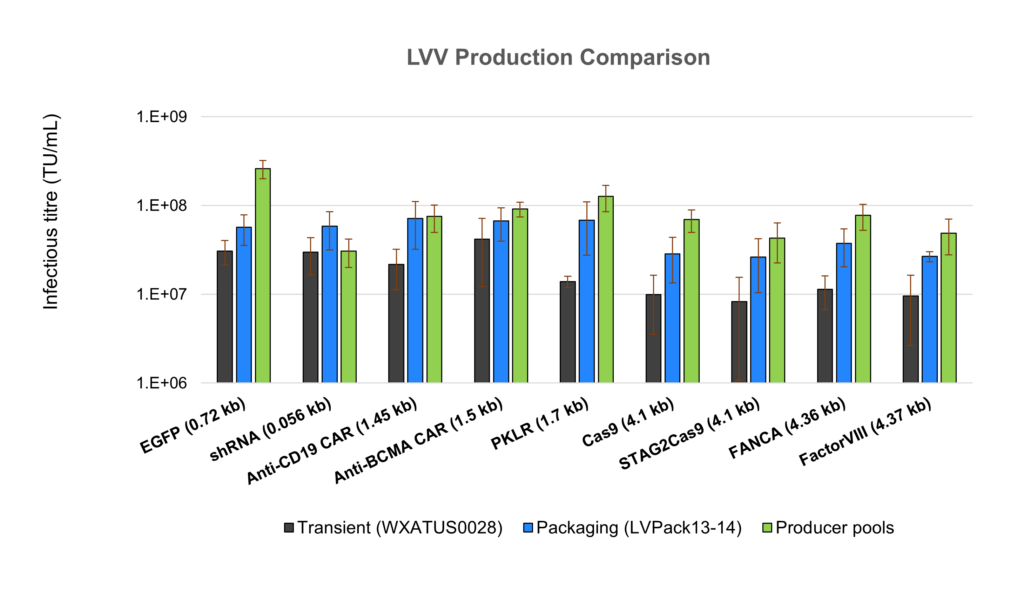
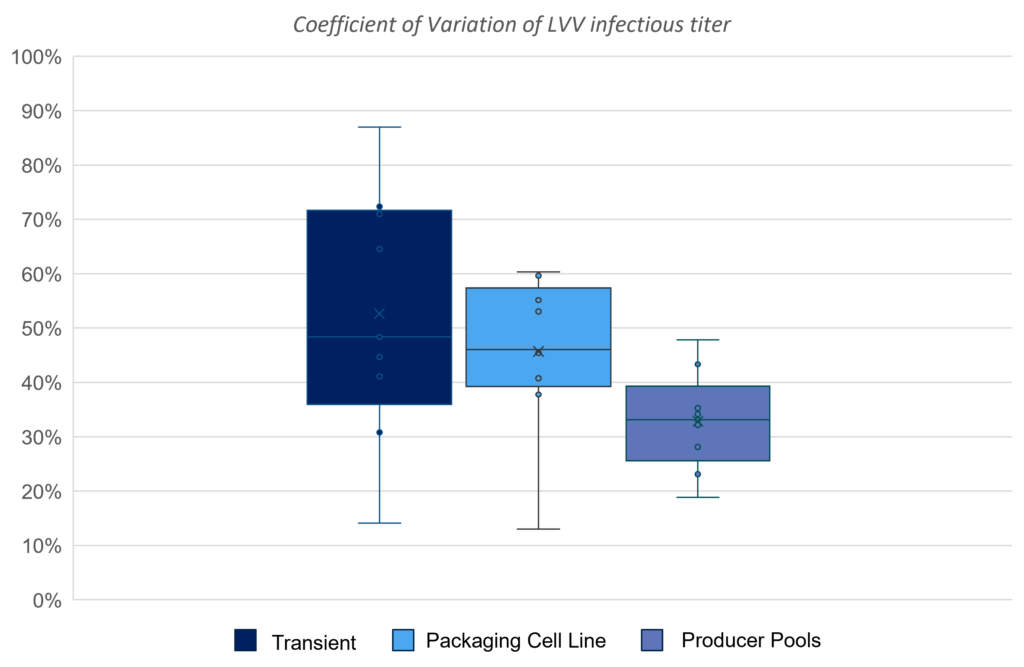
01. In-house Evaluation
If you are interested in exploring the application of custom XOFLX™ packaging or producer cell lines you can obtain a research-use-only license and receive the cell lines to test on your own.
For use during in-house manufacturing, you will need a clinical and commercial use license. Contact us for more information.