Service
Cell Line Engineering and Development
The team at OXGENE, an Advanced Therapies company, has a variety of expertise, starting materials, tools, and techniques to help you with customized cell line engineering and development projects. We also offer XOFLX™ stable packaging and producer cell lines for lentivirus vector manufacturing.
Engage with our experts
Cell Line Development Services and Solutions
The experts from OXGENE, an Advanced Therapy company, offer a variety of expertise and technologies to help you design and develop cell lines to support your pipeline.
Starting Materials
Suspension, Adherent, and Primary Cells
Expertise
Plasmid engineering and onboarding of new cell lines and plasmids
Engineering Techniques
Plasmid (random), transposon based stable transfection, Retroviral / lentiviral transduction
Monoclonality and Selection
Monoclonal cell line isolation and screening to identify best performer
Modification Techniques
Gene knock-down, knock-out, and knock-in
Required Documentation
Traceability, proof of clonality, stability testing
Discover XOFLX™

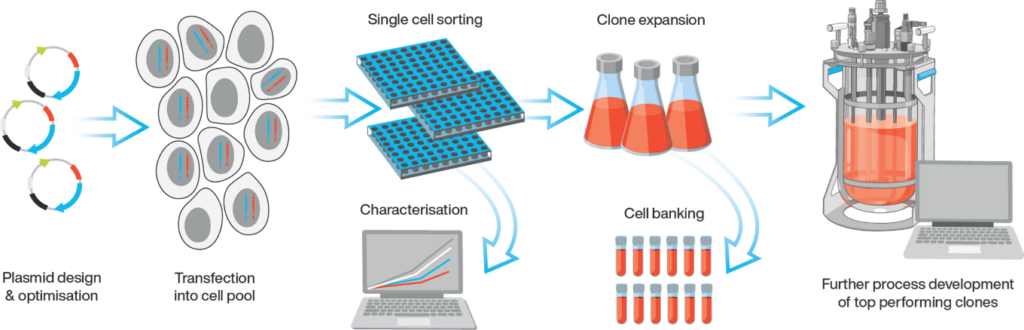
Streamlined and Efficient Workflow
We have experience handling different cell types as starting materials for your project, including adherent, suspension, and primary cells. Our plasmid engineering team will provide expertise and guidance on construct design as required. In addition, both viral and non-viral approaches are available for cell line engineering.
Non-viral strategies generate stable cell lines through random integration of plasmid(s) or transposon-based methods for transgene integration, via transfection reagents or nucleofection. The transposon-based method should result in a higher number of integrations per cell, and integrations directed to transcriptionally active loci, therefore increasing the transgene expression level in the engineered cells.
Viral approaches for cell line engineering are also available using a retroviral/lentiviral vector. We have in-house capabilities for designing and producing proprietary lentiviral vectors, which can transduce dividing and non-dividing cells for transgene integration. By manipulating the multiplicity of infection (MOI), we can generate cell lines with various levels of transgene copy numbers and expression levels.
Taking advantage of automated liquid handling platforms with integrated cell imagers, we can carry out high throughput monitoring and screening of monoclonal cell lines, providing accurate cell clonality proof and ensuring the shortest timeline for cell line development process

Quality Focused and Regulatory Friendly
Our cell line development services are aimed to deliver cell lines for R&D applications, as well as manufacturing platforms of your biologics. All our projects are ISO compliant and follow a rigid documentation methodology to provide a fully traceable process.
From cell line modification, clone screening and characterizing, all the way through to stability testing, the process is discussed with the customer, thoroughly customized, and documented. The full documentation package includes clonality reports, traceability tables and a report of testing for research cell bank release.
The image to the left shows the robotic-driven imaging software used to provide proof of clonality and monitor colony growth in the weeks post-sorting.

Clonal Suspension HEK293 Cell lines
Our clonal suspension HEK293 cell lines are validated for high-titre production of recombinant AAV (rAAV), lentiviral and adenoviral vectors. All cell lines, including engineered and media adapted derivatives, offer full traceability and grow in defined, animal component free media. Cell lines do not contain the SV40 large T antigen, which ensures regulatory compliance of derived products for in vivo applications.
In particular, Advanced Therapies developed a clonal HEK293 suspension cell line by doing suspension adaptation and single clone isolation and screening. 3000 clones were screened and the best cell line (WXATUS0028) demonstrated high growth rate & viability, low aggregation, and high viral productivity for LVV and AAV vectors.
The whole process was well documented and fully traceable, and the Drug Master File (DMF) is available for this cell line, supporting our customers in their regulatory submissions. The cell line does not include the SV40 large T antigen, reducing the oncogenic risk of the LVVs for in vivo therapies and overcoming the related regulatory hurdles.
Clonal Suspension HEK293TetR Cell Line
Our GMP compliant suspension HEK293TetR cell line is ideal for amplification of cytotoxic adenoviral vectors. We have demonstrated the functionality of this cell line through our partnership with Vaccitech, (now Barinthus Therapeutics) who are now using this cell line in clinical trials, and through partner CMOs.
In all cases, this animal component and antibiotic free cell line outperformed commercial alternatives. It also demonstrated improved scalability and full stability in the absence of antibiotics and allows optimisation of adenoviral production even for transgenes that reduce cell viability.
From Cell Line Development to GMP

For GMP applications, including banking of cell lines, we have a streamlined internal process to tech transfer to our sites in Philadelphia.
We also support our clients during tech and material transfer to their own manufacturing facilities or their CDMO of choice.