Service
Snapfast™ Custom and Off-the-Shelf Plasmids
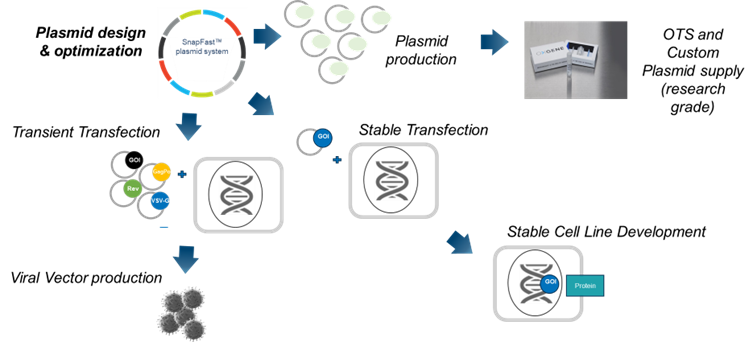
OXGENE’s SnapFast™ plasmids are versatile and efficient. These modular plasmids are designed to work like ‘molecular building blocks’, using a catalogue of well characterized DNA elements that can be easily and reliably inserted into specific locations within the plasmid to generate large numbers of custom constructs across a range of expression platforms. You can purchase off-the-shelf (OTS) plasmids or work with our team to develop custom plasmids.
Connect with our experts
Off-the-shelf plasmids and custom plasmid engineering services
The OXGENE team, a division of Advanced Therapies provides proven off-the-shelf plasmids and expert custom plasmid engineering services for use in cell and gene therapies.
SnapFast™ Off-the-Shelf Plasmids
Modular plasmids that enable increased editing accuracy and reduce turnaround times for LVV and AAV.
Starting materials
pSnapFast backbone, onboarding of desired backbone, in-house sequences or onboarding of client sequence or DNA prep
Process Engineering
Plasmid design, Synthesis, Cloning, Mutagenesis
Output and QC
Plasmid prep (from mini to giga-prep), Plasmid map, Quality Control (RE and sequencing) and Report of Testing
Plasmid Applications
Viral vectors, protein expression, GOI cassette optimization, DNA library design and construction
Plasmids Available in all Grades
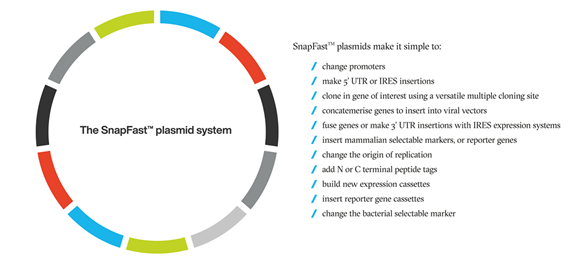
SnapFast™ Plasmid System
OXGENE’s SnapFast™ modular plasmids use a catalogue of characterized DNA elements that can be easily and reliably inserted into specific locations within the plasmid. This modularity enables increased editing accuracy and reduces turnaround times in plasmid engineering projects. Furthermore, our SnapFast™ transfer plasmids have been engineered to perform optimally with our AAV and LVV packaging systems for high quality viral vector production.
We have a wealth of experience in engineering plasmids for cell and gene therapy applications, which helps us offer guidance on construct design and provide excellent customer support. We offer comprehensive design consultation to optimize your gene of interest (GOI) construct, including codon and expression cassette optimization, to help enhance the manufacturability, potency, and safety of your viral vector. We also have robust production and quality control systems to ensure accurate and rapid plasmid engineering projects.
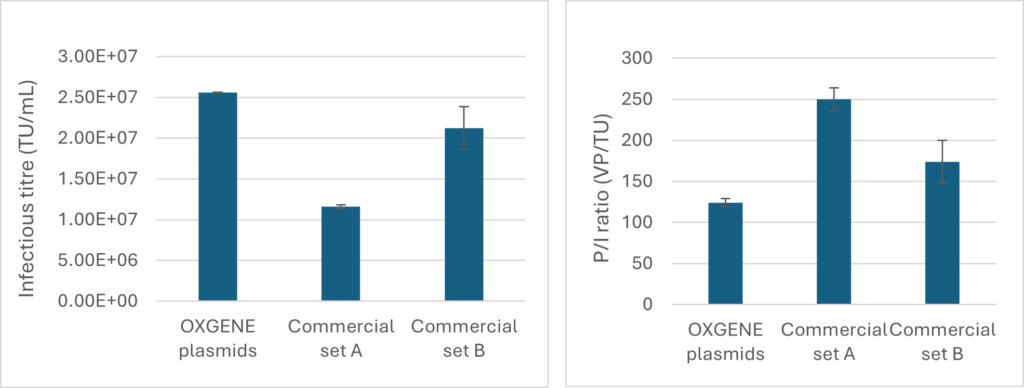
SnapFast™ Lentivirus Technology
OXGENE’s pSnapFast™ Lentivirus technology is a 3rd generation, self-inactivating (SIN) lentivirus packaging system.
The system is optimized for increased translation efficiency, it has low homology between expression cassettes for an improved safety profile and outperform other commercially available equivalents.
The figures to the right demonstrate the comparison of (A) infectious titres and (B) physical/infectious titre ratios (VP/TU) of in-house LVV plasmids in a fully transient production format compared to two equivalent commercially available plasmid sets. Fully transient production performed by transfection of adherent HEK293T cells. N = two biological replicates, error bars indicate standard deviation.
Infectious titration by flow cytometry-based measurement of per-centage EGFP positivity of transduced HEK293T cells. Physical titration by p24 ELISA.
Recent LVV Production Comparison Studies
Recently, we’ve replaced the wild-type Woodchuck hepatitis virus Posttranscriptional Regulatory Element (WPRE) to mut6 WPRE in our transfer plasmid backbone. This replacement still maintains the transcription-enhancing function of WPRE but eliminates the risk of expressing the toxic protein, hepatitis virus X protein, thus further improving the safety profile of the lentiviral vectors (LVVs) produced from our plasmid system. This improved safety feature is particularly important for lentiviral vector-based in vivo therapies.
Additionally, we expanded our offering of transfer plasmid backbones with commonly used human promoters driving the gene of interest (GOI) therapeutic cargo expression, making the custom cloning of the transfer plasmids easier and faster.
Our LVV production comparison studies show that different promoters have different impacts on LVV titres and GOI therapeutic cargo expression levels.
Performance of LV vectors produced from transfer plasmids where anti-CD19 CAR, anti-BCMA CAR and EGFP are driven by different promoters: EFS, EF-1ɑ and SFFV. Fully transient productions performed by transfection of suspension WXATUS0028 cells. N = three biological replicates in E125 shake flask. Infectious titration by ddPCR-based measurement of integrated vector copy number in transduced-then-passaged HT1080 cells.
SnapFast™ AAV Technology
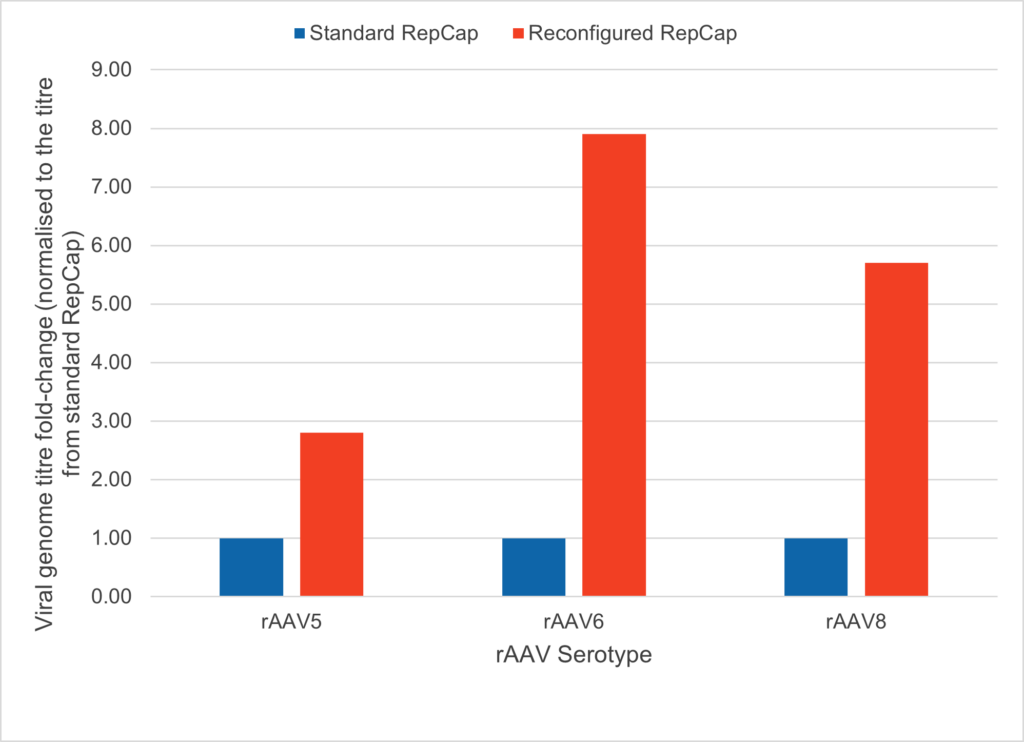
We have reconfigured the Rep and Cap genes to place Cap under the control of a strong CMV promoter, while Rep is expressed at a lower level via an EMCV internal ribosome entry site. This results in a higher viral titre, better per cell rAAV productivity and improved full:empty vector ratio. We’ve also improved adenoviral helper functionality by simplifying the Ad5 helper plasmid, which doesn’t contain any adenoviral hexon genes; an added benefit when it comes to regulatory approval.
In the figure you can see rAAV production in suspension HEK293 cell line in shake flasks. Using OXGENE’s reconfigured Rep-Cap plasmid (red) improves viral titre between two and eight fold, depending on AAV serotype.
Order off-the-shelf Plasmids
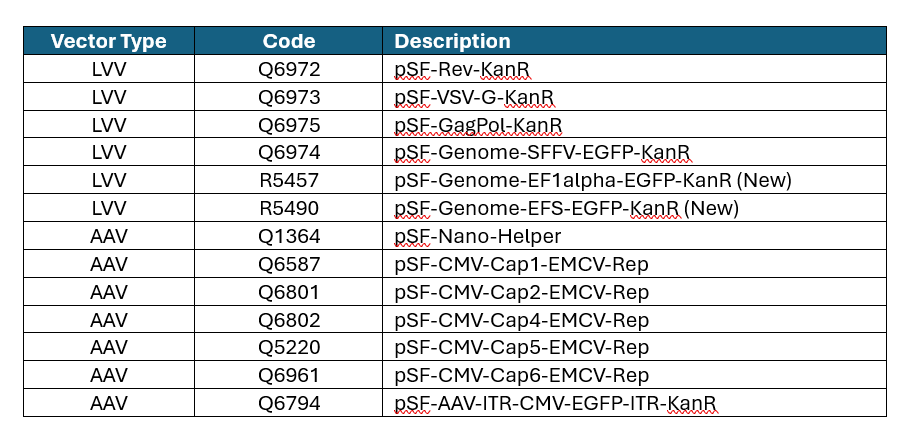
Here is a list of the off-the-shelf plasmids and some of our popular serotypes for both AAV and LVV. Please contact the team to place an order.