Service
Viral Vector Process Design & Development
OXGENE, an Advanced Therapies Company, offers early-stage screening and optimization of vector production systems, helping you identify the best elements for process development and manufacturing scale-up. We offer a comprehensive range of technologies and platforms, from transient transfection to cutting-edge solutions, such as TESSA® and XOFLX™. Our capabilities extend to analytical methodologies for the titration and quality control of viral vectors.
Get in touch with the team
Adeno-Associated Virus
Transient Transfection
Our transient expression platform comprises optimised proprietary AAV plasmids and a GMP-banked clonal suspension HEK293 cell line (add link: learn more about our cell line XXX).
OXGENE has also developed several modifications to enhance an AAV gene therapy production pipeline. One improvement is the re-engineering of the rep–cap plasmid, placing cap downstream of a strong cytomegalovirus promoter and using an internal ribosomal entry site (IRES) to initiate rep production.
We offer expert cloning and engineering services to customize and optimize our AAV plasmids for expression of your gene of interest, before process development and manufacture of your rAAV vectors at any grade and scale.
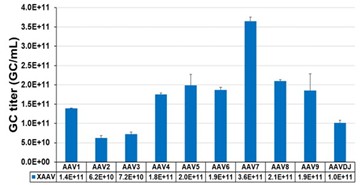
The figure to the right shows several AAV-serotypes produced using HEK293 suspension
platform and SnapFast AAV packaging plasmids for transient transfection process platform and SnapFast AAV packaging plasmids for transient transfection process.
Reconfiguration Improves Infectivity
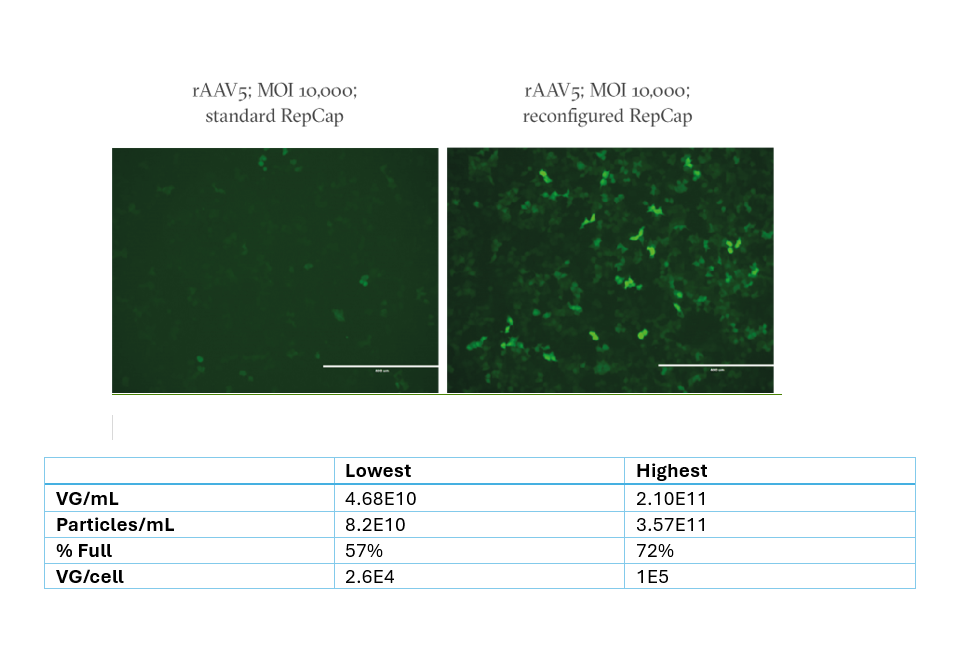
The figures to the right demonstrate how reconfiguration improves infectivity.
In the first image you can see how rAAV5 made using standard plasmids (left) infects HEK293 cells less efficiently than rAAV5 made using OXGENE’s reconfigured RepCap plasmid (right).
The table just below the image provides data from a third-party evaluation of OXGENE’s AAV5 plasmids using four different HEK293 cell lines. Highest and lowest results reported. VG titrated by ddPCR, physical particles by capsid ELISA.
TESSA® Scalable AAV Production

Lentivirus Vector Production
Transient Transfection
Our transient expression platform comprises optimized proprietary Lentivirus plasmids and a GMP-banked clonal suspension HEK293 cell line. By using our SnapFast™ Plasmid technology, we optimized a set of GagPol, Rev, Env (VSV-G) and LentiGenome plasmids with reduced homology for improved safety profile and high yields of infectious particles.
Our transient lentivirus production system enables consistent and reproducible results among batches and different HEK293 cell line platforms.
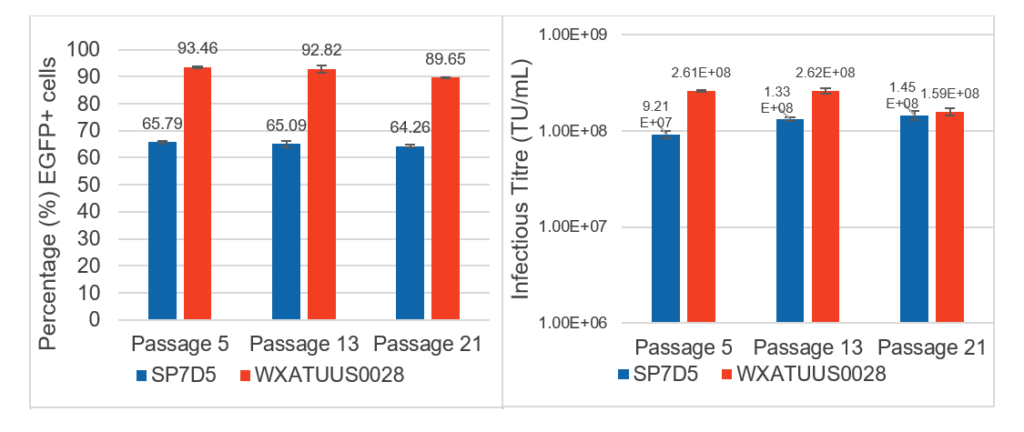
The figure to the right demonstrates the transfection efficiency (left) and average infectious titre (right) of OXGENE’s lentiviral packaging plasmids in both OXGENE’s (blue) and ATU’s (orange) clonal suspension HEK293 cell lines at both early, mid, and late passages.
Fully transient productions performed by transfection of suspension SP7D5 and WXATUS0028 cells in E125 shake flask. Transfection efficiency measured by flow cytometry at 72 hours post-transfection. Infectious titration by flow cytometry-based measurement of per-centage EGFP positivity of transduced HEK293T cells. N = two biological replicates, error bars indicate standard deviation.
Use of HEK293 Suspension Cell Line
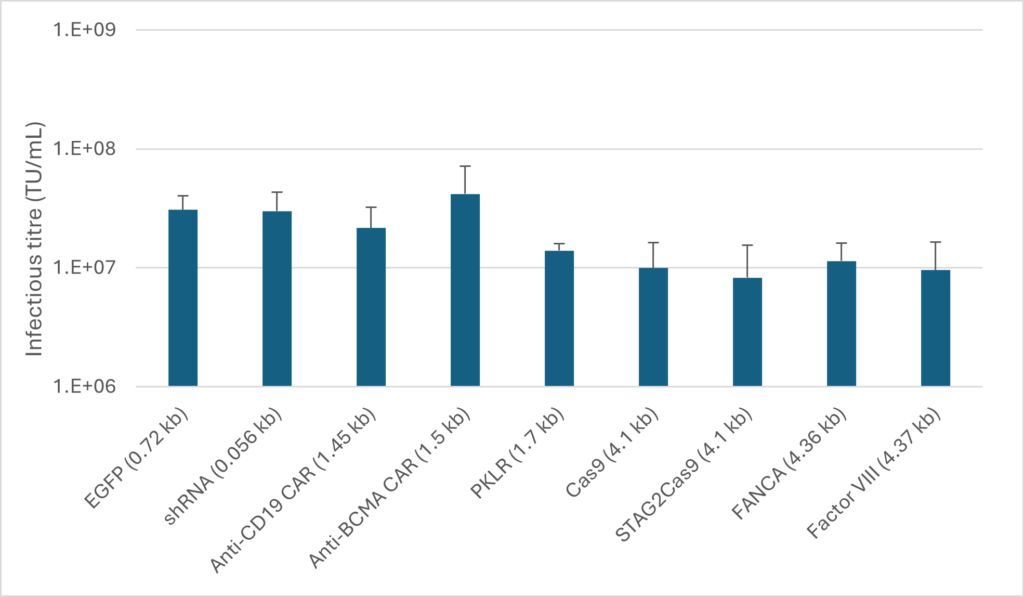
Our HEK293 suspension cell line has been used for producing a large number of batches of LV vectors containing various therapeutic transgenes for our customers, and our in-house testing also showed a good versatility of the cell line when producing a range of LV vectors encoding various sizes of GOIs with various sizes.
The figure to the right demonstrates LV vector production in HEK293 suspension cell line for various transgenes. All productions were performed in 24 deep-well plates. Infectious titration by qPCR measurement of integrated vector copy number in transduced HEK293T cells. N = four production replicates, with three transfection replicates per production. Error bars represent standard deviation.
Together with our standard process, we offer services of testing various promoters with clients’ GOIs therapeutic cargos to assess both LVV production titres and GOI cargo expression levels, and clients can also test the LVVs in the target cells for the relevant functions to ensure that the best promoter is selected for the LVV product.

XOFLX™ Stable Cell Line Technology for Scalable Lentivirus Vector Production
Viral Vector Processes Capabilities
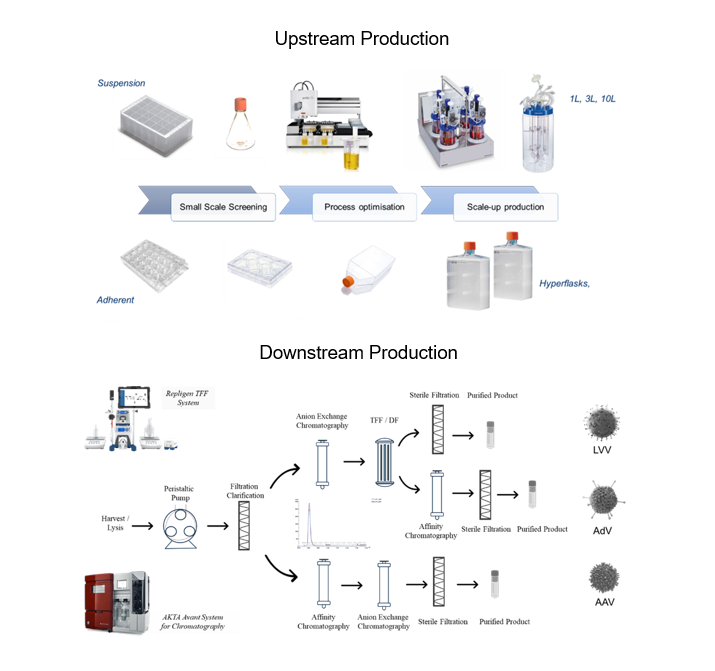
Industry Standards Adapted to Meet Your Program’s Needs
We understand practices of viral vector production are very specific to the therapeutic programs and innovative approaches of its developers.
Together with our standard technologies, our team strives in adapting expertise and capabilities to suit specific needs of your program and stage of development, we can on-board your materials, technologies and protocols to assess, select and scale-up your required process.
Typical viral vector processes (upstream and downstream) shows in the image to the right can be adapted to meet the specific requirements of your program.

Customized Project Work: Examples
We offer customized projects aimed to support you in the early-stage discovery and development of your program.
Below are some examples of custom projects we can undertake.
- Testing various promoters with clients’ GOIs therapeutic cargos to assess both viral vector production titres and GOI cargo expression levels
- Screening of various parameters at different scale and production modes (24-well plates, E125 flasks, AMBR15)
- Migrating from flask scale to Stirred Tank Reactor (E125, 1L, 3L and 10L STR)
- Optimizing downstream processing (chromatography, TFF, sterile filtration)
- Developing analytical methods (PCR-based detection methods, ELISA, Flow cytometry-based detection methods)
- Quality control and sequence verification such as:
- Nanopore GridION instrument permits five parallel runs
- P2 Solo permits use of PrometION flowcells for high fidelity applications
- Robust methods for sequencing:
- Plasmids
- AAV
- Adenovirus
- Amplicons
- Human cell lines
- In-house bioinformatics pipelines for data processing and alignment to a reference
- Bespoke AAV contamination pipeline and specialist expertise to investigate:
- Reverse packaging (genome transfer plasmid backbone)
- Packaging of Helper plasmid DNA
- Packaging of RepCap plasmid DNA
- Packaging of human cell line DNA